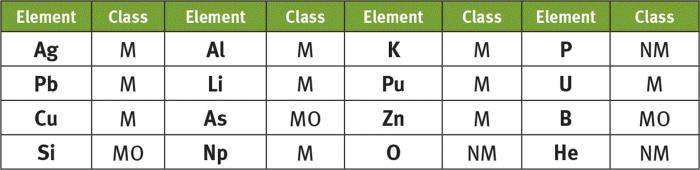
Periodic table given on mcat – Prepare to delve into the fascinating realm of chemistry with our comprehensive guide to the periodic table given on the MCAT. This essential tool provides a wealth of information about the elements, their properties, and their behavior, making it indispensable for aspiring medical professionals.
Throughout this guide, we will explore the history, organization, and significance of the periodic table. We will delve into the periodic trends and patterns that govern the elements and uncover the practical applications of this invaluable resource in various scientific fields.
Overview of the Periodic Table

The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. It is a powerful tool for understanding and predicting the behavior of elements and their compounds.
History of the Periodic Table
The development of the periodic table began in the 18th century with the work of scientists such as Antoine Lavoisier and Johann Wolfgang Döbereiner. However, it was not until 1869 that Dmitri Mendeleev published the first widely accepted periodic table, which organized the elements in order of increasing atomic weight and grouped them according to their chemical properties.
Organization of the Periodic Table
The periodic table is organized into 18 vertical columns, called groups, and 7 horizontal rows, called periods. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.
The elements in the periodic table are arranged so that elements with similar chemical properties are grouped together. For example, all of the alkali metals (Group 1) are highly reactive and form 1+ ions, while all of the halogens (Group 17) are highly reactive and form 1- ions.
Properties of Elements on the Periodic Table

The periodic table is a systematic arrangement of chemical elements based on their atomic number, electron configuration, and recurring chemical properties. Understanding the properties of elements and how they vary across the periodic table is crucial for comprehending their behavior in chemical reactions and predicting their reactivity.
Atomic Number, Electron Configuration, and Chemical Properties, Periodic table given on mcat
The atomic number of an element, which is the number of protons in its nucleus, determines its position on the periodic table. Elements in the same group (vertical column) share the same number of valence electrons, which are the electrons in the outermost energy level.
Valence electrons play a significant role in determining an element’s chemical properties, as they participate in chemical bonding.
For instance, alkali metals (Group 1) have one valence electron, making them highly reactive and prone to forming positive ions. In contrast, noble gases (Group 18) have a full valence electron shell, making them stable and unreactive.
Trends in Atomic Radius, Ionization Energy, and Electronegativity
Across the periodic table, several properties exhibit periodic trends. These trends include atomic radius, ionization energy, and electronegativity.
- Atomic Radius:Generally, atomic radius decreases from left to right across a period (horizontal row) and increases from top to bottom within a group. This is due to the increasing nuclear charge and the shielding effect of inner electrons.
- Ionization Energy:Ionization energy, the energy required to remove an electron from an atom, increases from left to right across a period and decreases from top to bottom within a group. The higher the ionization energy, the harder it is to remove an electron.
- Electronegativity:Electronegativity measures an atom’s ability to attract electrons. It generally increases from left to right across a period and decreases from top to bottom within a group. The higher the electronegativity, the more strongly an atom attracts electrons.
These trends help predict an element’s reactivity and behavior in chemical reactions. For example, elements with low ionization energy and high electronegativity, such as fluorine, are highly reactive and tend to form negative ions. Conversely, elements with high ionization energy and low electronegativity, such as sodium, are less reactive and form positive ions.
Groups and Periods on the Periodic Table

The periodic table is organized into vertical columns called groups and horizontal rows called periods. These groupings provide valuable information about the chemical properties of elements.
Groups
Groups, also known as families, are vertical columns on the periodic table. Elements within a group share similar chemical properties because they have the same number of valence electrons, which are the electrons in the outermost energy level. The group number indicates the number of valence electrons an element has.
Periods
Periods are horizontal rows on the periodic table. Elements within a period have the same number of electron shells. The period number indicates the highest energy level occupied by electrons in an element.
Arrangement of Elements
The arrangement of elements within groups and periods is as follows:
| Group | Period | Element |
|---|---|---|
| 1 | 1 | Hydrogen |
| 2 | 1 | Helium |
| 1 | 2 | Lithium |
| 2 | 2 | Beryllium |
| … | … | … |
Periodic Trends and Patterns
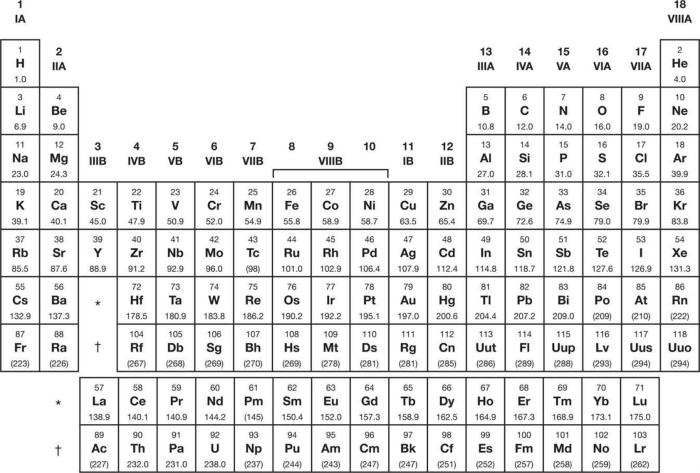
The periodic table is not merely a static arrangement of elements; it reveals dynamic trends and patterns in their properties. These trends provide valuable insights into the behavior of elements and allow us to predict the properties of unknown elements.
Across periods (horizontal rows), elements generally exhibit a gradual change in properties from left to right. This is due to the increase in atomic number, which corresponds to an increase in the number of protons and electrons.
Down groups (vertical columns), elements share similar chemical properties due to having the same number of valence electrons. However, their physical properties may vary significantly due to differences in atomic size and mass.
Metallic Character
Metallic character refers to the tendency of an element to exhibit metallic properties such as luster, malleability, and conductivity. In general, metallic character decreases from left to right across a period and increases from top to bottom down a group.
Alkali metals (Group 1) are the most metallic elements, while noble gases (Group 18) are the least metallic.
Nonmetallic Character
Nonmetallic character refers to the tendency of an element to exhibit nonmetallic properties such as dullness, brittleness, and low conductivity. In general, nonmetallic character increases from left to right across a period and decreases from top to bottom down a group.
Halogens (Group 17) are the most nonmetallic elements.
Metalloids
Metalloids are elements that exhibit properties of both metals and nonmetals. They are located along a diagonal line running from boron (B) to polonium (Po) on the periodic table.
Metalloids have intermediate electrical conductivity and can form alloys with both metals and nonmetals.
Predicting Properties of Unknown Elements
The periodic trends allow us to predict the properties of unknown elements based on their position on the periodic table.
The periodic table given on MCAT is a valuable resource for understanding the chemical elements and their properties. To test your knowledge of the periodic table, try solving the leaves on the side crossword . This puzzle challenges you to identify elements based on their atomic numbers, symbols, and names.
By completing the crossword, you’ll reinforce your understanding of the periodic table and expand your chemistry knowledge.
For example, if we discover a new element located in Group 15 and Period 4, we can predict that it will be a solid nonmetal with intermediate electrical conductivity.
Applications of the Periodic Table

The periodic table is a powerful tool that has revolutionized our understanding of the natural world. It has numerous applications in various fields of science, including chemistry, physics, and materials science.
One of the most important applications of the periodic table is in understanding chemical bonding. The periodic table helps us predict the type of bonds that will form between different elements based on their position in the table. This knowledge is essential for understanding the structure and properties of compounds.
Predicting Chemical Reactions
The periodic table can also be used to predict chemical reactions. By understanding the reactivity of different elements, we can predict the products of a reaction and the conditions under which it will occur. This information is invaluable for chemists who are developing new drugs, materials, and other products.
Designing New Materials
The periodic table is also a valuable tool for designing new materials. By understanding the properties of different elements, we can create materials with specific properties, such as strength, durability, and conductivity. This knowledge has led to the development of new materials that are used in a wide range of applications, from aerospace to medicine.
The periodic table is a powerful tool that has had a profound impact on our understanding of the natural world. It is a testament to the power of human ingenuity and the beauty of science.
FAQ Corner: Periodic Table Given On Mcat
What is the purpose of the periodic table?
The periodic table organizes and categorizes chemical elements based on their atomic number, electron configuration, and recurring chemical properties, providing a comprehensive overview of their behavior and relationships.
How is the periodic table organized?
The periodic table is arranged in 18 vertical columns (groups) and 7 horizontal rows (periods). Elements within the same group share similar chemical properties, while elements within the same period have the same number of electron shells.
What are some practical applications of the periodic table?
The periodic table finds applications in various fields, including chemistry, physics, and materials science. It aids in predicting chemical reactions, designing new materials, and understanding the behavior of elements in different compounds and environments.